Physics of Intracellular Organization
Ivo Telley
The research group is a multidisciplinary team interested in the physical aspects of intracellular organization.
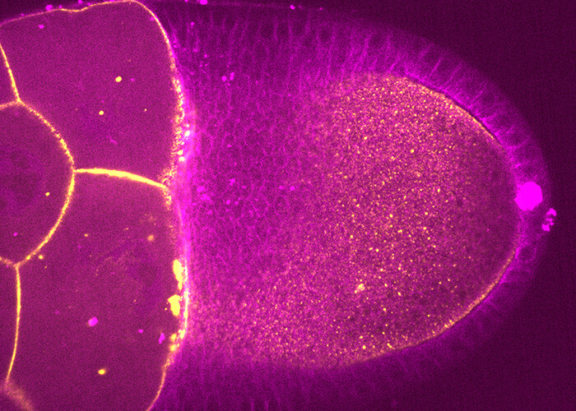
As a model system, they study the earliest stage of Drosophila development, from oogenesis to the mature egg to fertilization and blastoderm cleavages. On one hand, they focus on pronuclear fusion in the fertilized egg and how the syncytial embryo defines the inter-nuclear distance between rapid mitotic divisions. On the other hand, researchers study the physical and biochemical rules determining oocyte polarity.
They use a variety of approaches from chemistry, molecular biology, genetic engineering, micromechanics and optical microscopy.
Their core experimental platform is a cytoplasmic explant assay using single oocytes, mature eggs or early embryos.
Funding

![]()
Projects
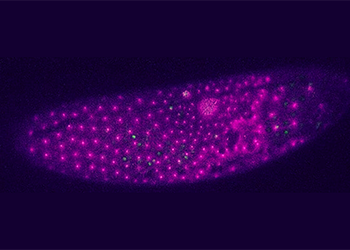
The intracellular positioning of the nucleus has gained substantial interest among biologists due its relevance in cell cycle, differentiation, migration, and polarity. Abnormal positioning has been related to cell and tissue function deficiency and severe defects in embryogenesis. Membrane linkers and cytoskeletal and molecular motor dynamics are essential factors for nuclear movement. However, understanding the coordination and the quantification of force generating elements for nuclear positioning are current challenges. In this project, we investigate time-resolved nuclear distribution starting at the earliest stage of Drosophila embryo development to understand causality and regulation of nuclear positioning. We infer on how predefined external factors (e.g. neighbor nuclei, cytoplasmic volume, geometry) influence the dynamics of the cytoskeletal machinery surrounding a nucleus, with focus on microtubules, actin, associated molecular motors and linking proteins.
Fertilization is a vital process as it lies at the heart of animal reproduction. Because fertilization happens deep inside the egg, which is carried inside a living organism and filled with diffractive yolk, time-lapse light microscopy has been challenging. Most of the existing knowledge on insect fertilization was obtained from the analysis of fixed and immunostained eggs. Thus, we lack important temporal and dynamic information of molecular and cellular processes such as chromosome and cytoskeletal dynamics. The main goal of this project is to develop a reconstitution assay of Drosophila melanogaster fertilization enabling live microscopy. Our assay will open new avenues to study chromosomal dynamics during fertilization enabling more detailed insight, for example, into bacteria transmission and cytoplasmic incompatibility (CI).
Publications
- Ana Milas, Jorge de-Carvalho & Ivo A. Telley (2022) Follicle cell contact maintains main body axis polarity in the Drosophila melanogaster oocyte. Journal of Cell Biology 222 (2):e202209052
- Margarida Araújo, Alexandra Tavares, Diana V Vieira, Ivo A Telley and Raquel A Oliveira (2022) Endoplasmic reticulum membranes are continuously required to maintain mitotic spindle size and forces. Life Science Alliance
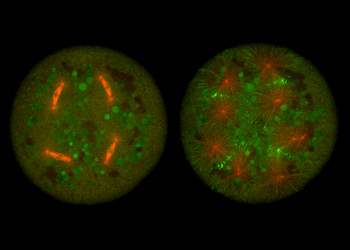
- Jorge de-Carvalho, Sham Tlili, Lars Hufnagel, Timothy E. Saunders, Ivo A. Telley (2022) Aster repulsion drives short-ranged ordering in the Drosophila syncytial blastoderm. Development 149(2)
- Ojas Deshpande, Jorge de-Carvalho, Diana V. Vieira and Ivo A. Telley (2021) Astral microtubule cross-linking safeguards uniform nuclear distribution in the Drosophila syncytium. The Journal of Cell Biology 221 (1)
- Nabais C, Pessoa D, de-Carvalho J, van Zanten T, Duarte P, Mayor S, Carneiro J, Telley IA, Bettencourt-Dias M. (2021) Plk4 triggers autonomous de novo centriole biogenesis and maturation.. J Cell Biol. 220(5):e202008090
- Lv Z, de-Carvalho J, Telley IA, Großhans J. (2021) Cytoskeletal mechanics and dynamics in the Drosophila syncytial embryo.. J Cell Sci. 134(4):jcs246496
- Jorge de-Carvalho, Sham Tlili, Lars Hufnagel, Timothy E. Saunders, Ivo A. Telley (2020) Aster repulsion drives local ordering in an active system.. bioRxiv
- Ojas Deshpande, Jorge de-Carvalho, Diana V. Vieira, Ivo A. Telley (2019) Astral microtubule crosslinking by Feo safeguards uniform nuclear distribution in the Drosophila syncytium. bioRxiv
- de-Carvalho J, Deshpande O, Nabais C, Telley IA. (2018) A cell-free system of Drosophila egg explants supporting native mitotic cycles.. Methods Cell Biol. 144:233-257
- Silva A.M., Osório D.S., Pereira A.J., Maiato H., Pinto I.M., Rubinstein B., Gassmann R., Telley I.A., Carvalho A.X. (2016) Robust gap repair in the contractile ring ensures timely completion of cytokinesis.. J Cell Biol. 215(6):789-799
- Telley I.A., Gaspar I., Ephrussi A., Surrey T. (2013) A single Drosophila embryo extract for the study of mitosis ex vivo.. Nat Protoc. 8(2):310-324
- Telley I.A., Gaspar I., Ephrussi A., Surrey T. (2012) Aster migration determines the length scale of nuclear separation in the Drosophila syncytial embryo.. J Cell Biol. 197:887-895
- Telley I.A., Bieling P., Surrey T. (2011) Reconstitution and quantification of dynamic microtubule end tracking in vitro using TIRF microscopy.. Methods Mol Biol. 777:127-45
- Bieling P., Telley I.A., Surrey T. (2010) A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps.. Cell 142:420-432
- Bieling P.*, Telley I.A.*, Hentrich C., Piehler J., Surrey T. (2010) Fluorescence microscopy assays on chemically functionalized surfaces for quantitative imaging of microtubule, motor and +TIP dynamics.. Methods Cell Biol. 95:549-574
- Telley I.A., Bieling P., Surrey T. (2009) Obstacles on the microtubule reduce the processivity of Kinesin-1 in a minimal in vitro system and in cell extract.. Biophys J. 96:3341-3353
- Sung H., Telley I.A., Papadaki P., Ephrussi A., Surrey T., Rørth P. (2008) Drosophila Ensconsin promotes productive recruitment of Kinesin-1 to microtubules.. Dev Cell. 15:866-876
- Bieling P., Kandels-Lewis S., Telley I.A., van Dijk J., Janke C., Surrey T. (2007) CLIP-170 tracks groing microtubule ends by dynamically recognizing composite EB1/tubulin binding sites.. J Cell Biol. 183:1223-1233
- Telley I.A., Denoth J., Stüssi E., Pfitzer G., Stehle R. (2006) Half-sarcomere dynamics in myofibrils during activation and relaxation studied by tracking fluorescent markers.. Biophys J. 90(2):514-530