There and back again: how neurons make room for growth in a developing organ
Researchers at the Instituto Gulbenkian de Ciência (IGC, Oeiras) and Max Planck Institute of Cell Biology and Genetics (MPI-CBG, Dresden) identified a new mechanism that exposes some of the multitasking abilities embryos need to build a functional retina (Fig. 1), the sensory part of the central nervous system responsible for vision. The discovery, published in Nature, provides an important contribution to understanding how healthy organs are built, which is ultimately critical for understanding how organ malformations that affect 8 million newborns every year arise.
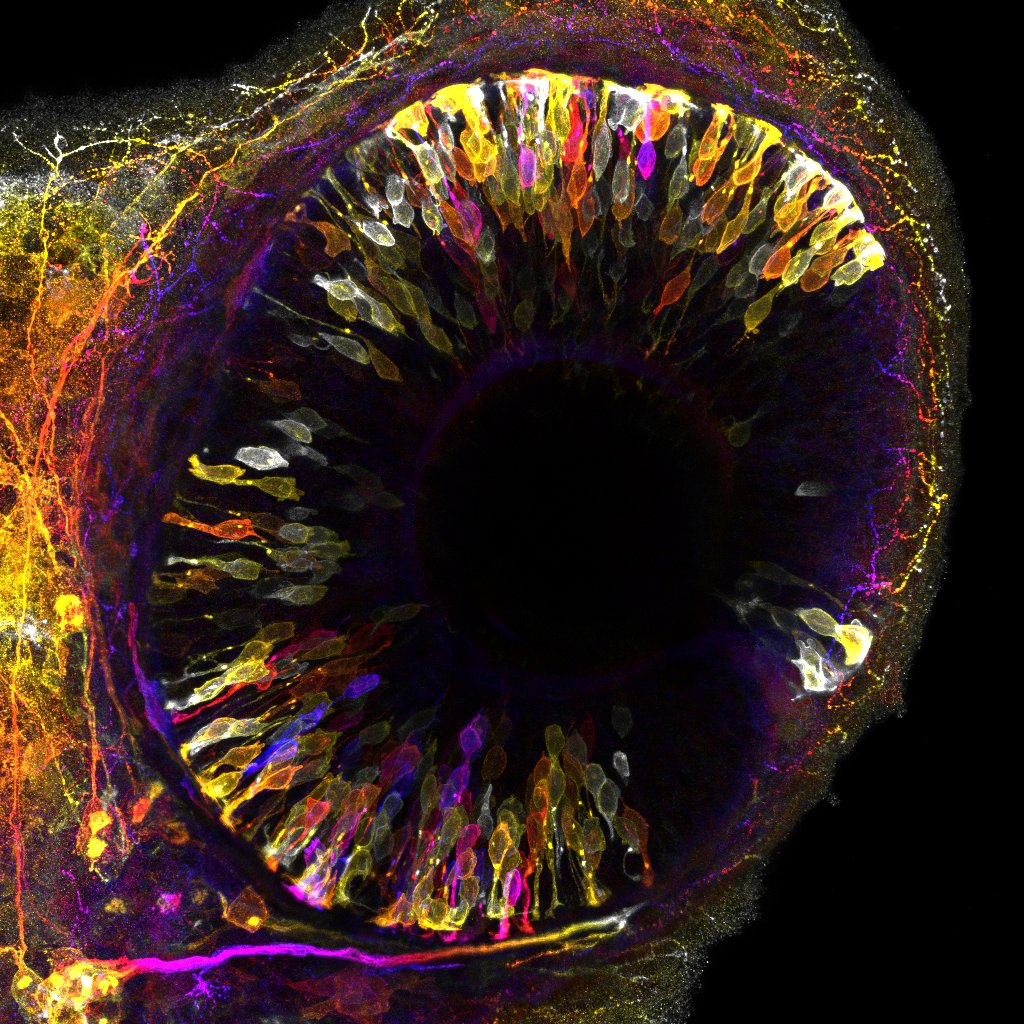
Photoreceptor neurons undergo transient relocation at the peak of retinal growth to prevent spatial competition. Developing retina of zebrafish embryo at 42 hours post-fertilization. Emerging photoreceptor
neurons labeled by transgenic reporter color-coded for depth. ©Mauricio Rocha-Martins, IGC 2023.
To function correctly, organs require a precise number of cells and a functional architecture established during embryogenesis. Embryos are proficient multitaskers; they grow, acquire shape and functional architecture all at once. Despite much research on embryo development, scientists still need to fully grasp how embryos orchestrate all these tasks in space and time to ensure the formation of healthy organs.
This was the study’s central question led by Caren Norden (group leader) and Mauricio Rocha-Martins (postdoctoral researcher). The research team, which also involved computer scientists, used cutting-edge technology to explore how the vertebrate retina copes with the challenges of growing profusely while, at the same time, remodeling tissue architecture. The retina of zebrafish embryos and human retinal organoids—mini-retina-like structures in a dish grown from human cells—were used as model systems because they both offer unique advantages due to their small size and high translucency, allowing real-time observation of tissue organization and growth. Advanced microscopy techniques, such as light-sheet microscopy and state-of-the-art image restoration based on deep learning, provided unprecedented insight into the cellular behaviors involved.
The researchers observed that an entire population of neuron photoreceptors temporarily relocates away from the zone of the tissue where they reside and must fulfill their function (Fig. 1). This active movement creates space for incoming progenitor cells that divide in this area and thereby produce more cells that later contribute to the neuronal retina. Blockage of the movements of photoreceptors leads to congestion, forcing progenitor cells to divide in the wrong place, which in turn causes tissue malformation. Thus, by transiently moving away, neurons avoid interference with progenitor cells to ensure harmonious organ development.
To Mauricio Rocha-Martins, the study’s first author, “This is a curious migration phenomenon, in which neurons move away just to move back then, ending up where they started. It highlights that neuronal migration, as opposed to previously believed, does not only move neurons to their correct location but can also play a direct role in the coordination of organ development”.
The implications of this research extend beyond the field of retinal development. Simultaneous growth and acquisition of functional architecture is a hallmark of most developing organs; the new findings offer the possibility to investigate whether other developing organs employ similar strategies. Moreover, it is known that defects in neuronal migration can cause severe brain malformations in humans. The findings that failed migration of neurons can have harmful consequences beyond the positioning of neurons points to the importance of examining the interactions between cells to understand the causes of human developmental disorders fully.
The study was supported by the MPI-CBG, the FCG-IGC, the German Research Foundation (NO 1069/5-1), and an ERC Consolidator Grant (H2020 ERC-2018-CoG-81904).
Read paper